Abstract
Background: CD19-targeted CAR-T cells have transformed the treatment of relapsed/refractory B-cell malignancies. However, relapse occurs in over 50% of patients treated with commercial CD19 directed CAR-T due to antigen escape from selective immune pressure either on the target antigen or due to exhaustion and lack of persistence of CAR-T cells. Hence there is an unmet need for next-generation CAR-Ts that have broad antigen recognition and novel costimulatory domains to promote survival and decrease exhaustion of CAR-Ts. Targeting three B-cell antigens simultaneously is a novel approach to prevent antigen-negative relapse while inclusion of the OX40 domain will promote CAR-Ts persistence and expansion. Whether targeting three commonly expressed antigens is feasible or safe and will result in durable remissions is not known. We hypothesize that this trispecific targeting of CD19/20/22 CAR-T construct will be safe and effective in patients with relapsed, refractory B-cell malignancies.
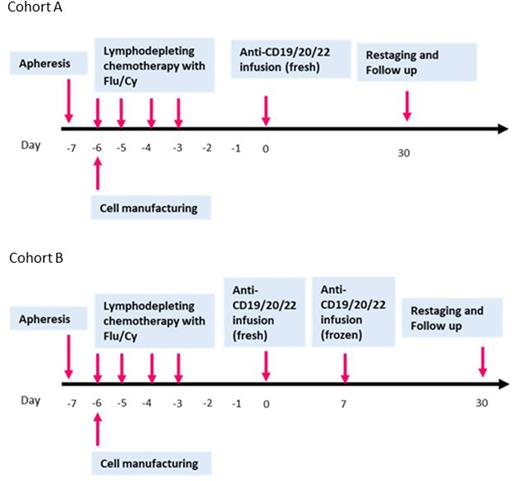
Study Design and Methods: This parallel group phase 1 study follows a 3+3 phase 1 design to determine the safety of the infusion of point-of-care manufactured fresh CAR-T cells targeting CD19/20/22 and to find the recommended phase II dose for this cellular therapy. CAR-T cells will be manufactured over 6 days. Patients will be enrolled in one of the two cohorts: Cohort A (single infusion): Non-Hodgkin's Lymphoma (NHL) with lesions <= 5 cm; Indolent lymphomas; B-PLL (B-Pro lymphocytic leukemia); Chronic Lymphocytic Leukemia -CLL (without Richter's transformation), Cohort B (split infusion): B-ALL (B-Acute lymphoblastic leukemia); Lymphoid blast crisis of Chronic myeloid leukemia; CLL (with Richter's transformation); NHL with lesions > 5 cm and/or lymphoblastic lymphoma, or NHL with circulating lymphoma cells. Patients who are CAR-naïve will be enrolled in this study. The study involves infusion of anti-CD19/20/22 CAR-T cells administered after an immunosuppressive conditioning regimen of fludarabine and cyclophosphamide. Cyclophosphamide 60 mg/kg will be infused intravenously over 60 minutes on day -6. Fludarabine 25 mg/m2 will be infused intravenously daily over 30 minutes on days -5 to -3. In each group, patients will be enrolled sequentially to each dose level, starting with dose level 1. Three CAR-T dose-levels: 5x105, 1x106 & 2x106 cells/kg/dose will be explored to determine MTD. For cohort B patients, the total CAR-T cell dose infused, will be divided in two doses, dose 1 (day 0) is 40% of the total dose and dose 2 (day 7) is 60% of total dose. The minimum number of subjects enrolled in the complete study will be 18 (9 per cohort) and the maximum number in dose escalation will be 36 (18 per cohort). Patients in each cohort will be monitored for 30 days before enrollment of next patientTwo patients have been enrolled in the trial and received fresh infusion of Anti CD19/20/22 CAR-T cells and accrual is ongoing. Updates on the initial cohort of patients will be presented at the meeting.
ClinicalTrials.gov NCT: NCT05418088
Disclosures
Vasu:Boehringer Ingelheim: Other: Travel support; Seattle Genetics: Other: Travel support. Schneider:Lentigen Technology, a Miltenyi Biotec Company: Current Employment. de Lima:Amgen: Consultancy; Incyte: Consultancy; Pfizer: Consultancy, Research Funding; Celgene: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal